Abstract
Background: Rigorous studies define the use of chronic transfusion therapy (CTT) for secondary stroke prevention in children with sickle cell anemia (SCA). Limited evidence informs the use of indefinite CTT for children with SCA and a history of stroke when they enter adult care. Often, adults with a history of childhood stroke are treated by extrapolation of pediatric practice and continue CTT. However, the risk of recurrent stroke in these adults is unknown. In this retrospective study, we describe outcomes of adults with a history of SCA and childhood stroke and compare them to adults without a history of childhood stroke.
Methods: The Johns Hopkins University Institutional Review Board approved this study. The inclusion criteria were: SCA diagnosis, ≥ 18 years old, received adult care at Johns Hopkins Hospital (JHH), and documentation of a stroke via diagnosis code or mention in hematology clinic notes prior to the age of 18 or no stroke in childhood. Subjects without a history of stroke in childhood were matched based on age, gender, and genotype.
For each subject, we performed a systematic chart review extending from their initial encounter with hematology care at JHH (pediatric or adult, whichever was available) to the final visit in the electronic medical record. Data collection spanned from January 1990 to February 2022. Variables collected include demographic data, SCA morbidities, and CTT treatments. We reported descriptive statistics for the subjects with a history of childhood stroke. Then we compared subjects with and without childhood stroke using the Mann Whitney U test for continuous outcomes and Fischer's exact test for categorical outcomes. We performed data analysis with Stata/SE Version 16.1.
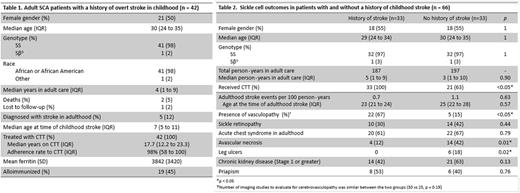
Results: There were 42 subjects with SCA and a history of childhood stroke (Table 1). In this group, the median age at the time of the study was 30 years (IQR 24 to 35 years). The median age at the time of childhood stroke was 7 years (IQR 5 to 11 years), and all subjects received CTT for secondary stroke prevention. Subjects received a median of 17.7 years of CTT (IQR 12.2 to 23.3 years). The median annual pretransfusion hemoglobin S for this group was 31% (IQR 24 to 39%), which suggests adherence to CTT. The median ferritin was 3842 ng/dL (SD 3842); 45% were alloimmunized. Five subjects with a history of childhood stroke had a stroke in adulthood: one subject (age 24) had discontinued CTT, two subjects (age 21, 23) were intermittently adherent to CTT and stroke occurred at a hemoglobin S of 49% in one subject (other unrecorded), and two subjects (ages 21, 22) received regular CTT and presented with a hemoglobin S of 14% and 24% respectively.
Comparison of subjects with and without a history of childhood stroke are shown (Table 2). More subjects with a history of childhood stroke received CTT than those without (33 (100%) vs 21 (63%), p = 0.0001). We observed 0.7 strokes per 100 person·years in subjects with a history of childhood stroke and 1.1 strokes per 100 person·years in subjects without a history of childhood stroke. These were not different (p = 0.63).
A comparison of SCD complications between those with and without a history of childhood stroke identified that adults on CTT because of childhood stroke had less avascular necrosis (AVN) (4 (12%) vs 12 (42%), p = 0.01) and leg ulcers (0 vs. 6 (18%), p = 0.02) than those without a history of childhood stroke.
Conclusions: Among 42 adults with a history of childhood stroke, recurrent strokes occurred in 12% and in a comparable number of adults without a history of childhood stroke. This finding suggests that continuing CTT in adult care may reduce the risk of recurrent stroke to that of a population without a history of childhood stroke. Here, we find that adults with a history of childhood stroke on CTT have lower rates of AVN and leg ulcers, which suggests that CTT may reduce the risk of developing these complications.
Larger, prospective studies are needed to define the extent to which CTT reduces recurrent stroke in adults with a history of childhood stroke. This evidence is necessary to inform decisions to continue CTT in adulthood because iron overload associated with CTT can be morbid and the burden of CTT visits may lead to missed work, lost wages, and childcare challenges. Further studies may also enhance decisions during pediatric care to direct children with history of stroke towards curative therapy.
Disclosures
Pecker:GBT: Research Funding; Novo Nordisk: Consultancy; Global Blood Therapeutics: Consultancy. Lanzkron:bluebird bio: Other: Adjudication committee, Research Funding; Novartis: Consultancy, Research Funding; Glycomimetics: Consultancy; Imara: Research Funding; GBT: Research Funding; Novartis: Research Funding; Takeda: Research Funding; CSL-behring: Research Funding; HRSA: Research Funding; PCORI: Research Funding; Teva: Current equity holder in private company; Pfizer: Consultancy, Current equity holder in private company; Novo Nordisk: Other: Adjudication committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal